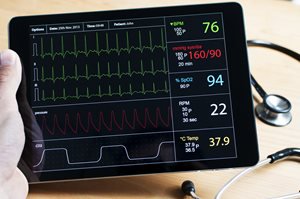
FDA, with academic and community partners, launches first pediatric ECG warehouse
 Clinical TrialsDiagnostics/IVDsMedical DevicesNorth AmericaPharmaceuticalsRegulatory Intelligence/PolicyResearch, Design and Development
Clinical TrialsDiagnostics/IVDsMedical DevicesNorth AmericaPharmaceuticalsRegulatory Intelligence/PolicyResearch, Design and Development Clinical TrialsDiagnostics/IVDsMedical DevicesNorth AmericaPharmaceuticalsRegulatory Intelligence/PolicyResearch, Design and Development
Clinical TrialsDiagnostics/IVDsMedical DevicesNorth AmericaPharmaceuticalsRegulatory Intelligence/PolicyResearch, Design and Development