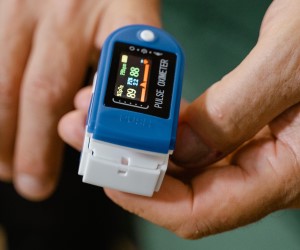
FDA advisors want standards, labeling to address racial disparities with pulse oximeters
 Advertising, Promotion and LabelingClinical TrialsComplianceDiagnostics/IVDsEthicsHealth Authority meeting and communication strategyNorth AmericaQuality Assurance and ControlRegulatory Intelligence/PolicyResearch, Design and Development
Advertising, Promotion and LabelingClinical TrialsComplianceDiagnostics/IVDsEthicsHealth Authority meeting and communication strategyNorth AmericaQuality Assurance and ControlRegulatory Intelligence/PolicyResearch, Design and Development