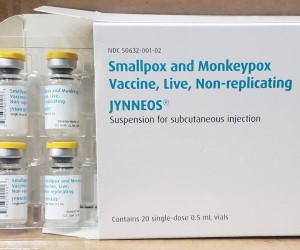
FDA issues Jynneos EUA to stretch monkeypox vaccine supply
 Biologics/ biosimilars/ vaccinesClinical TrialsNorth AmericaPharmaceuticalsRegulatory Intelligence/PolicySupply Chain Management
Biologics/ biosimilars/ vaccinesClinical TrialsNorth AmericaPharmaceuticalsRegulatory Intelligence/PolicySupply Chain Management Biologics/ biosimilars/ vaccinesClinical TrialsNorth AmericaPharmaceuticalsRegulatory Intelligence/PolicySupply Chain Management
Biologics/ biosimilars/ vaccinesClinical TrialsNorth AmericaPharmaceuticalsRegulatory Intelligence/PolicySupply Chain Management