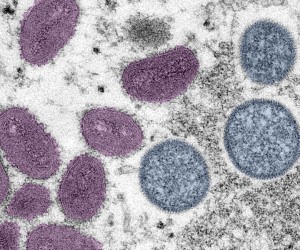
FDA issues draft guidance on mpox drug development
 Clinical TrialsComplianceEthicsNorth AmericaPharmaceuticalsPreclinical studyRegulatory Intelligence/PolicyResearch, Design and Development
Clinical TrialsComplianceEthicsNorth AmericaPharmaceuticalsPreclinical studyRegulatory Intelligence/PolicyResearch, Design and Development Clinical TrialsComplianceEthicsNorth AmericaPharmaceuticalsPreclinical studyRegulatory Intelligence/PolicyResearch, Design and Development
Clinical TrialsComplianceEthicsNorth AmericaPharmaceuticalsPreclinical studyRegulatory Intelligence/PolicyResearch, Design and Development