Expert: Companies should embrace ‘sustainable compliance’ to avoid 483s, warning letters
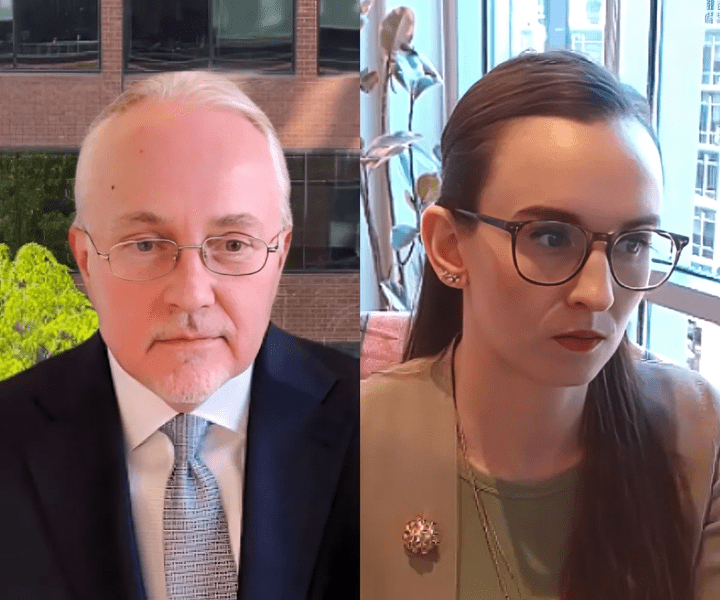 Biologics/ biosimilars/ vaccinesCDERComplianceGMPHealth Authority meeting and communication strategyPharmaceuticalsRegulatory Intelligence/PolicyUnited States
Biologics/ biosimilars/ vaccinesCDERComplianceGMPHealth Authority meeting and communication strategyPharmaceuticalsRegulatory Intelligence/PolicyUnited States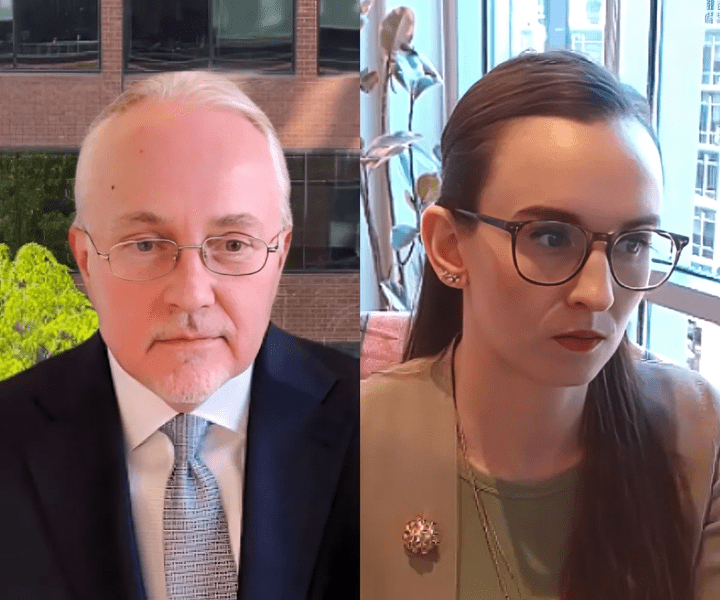 Biologics/ biosimilars/ vaccinesCDERComplianceGMPHealth Authority meeting and communication strategyPharmaceuticalsRegulatory Intelligence/PolicyUnited States
Biologics/ biosimilars/ vaccinesCDERComplianceGMPHealth Authority meeting and communication strategyPharmaceuticalsRegulatory Intelligence/PolicyUnited States